With the growing global demand for renewable energy and the reduction of fossil fuel reserves, the development of efficient, low-cost and safe energy storage technology has become an urgent problem to be solved. Sodium-ion batteries (SIBs) have shown great potential in large-scale energy storage applications due to their abundant sodium resources and low production costs. Sodium-ion batteries not only inherit many advantages of lithium-ion batteries, but also overcome the problems of lithium resource shortage and high cost. However, SIBs still face many challenges when used in a wide temperature range. This article will introduce the design principles, failure mechanisms, basic chemistry and safety issues of sodium-ion batteries in detail.
Key words:
1. Design principles
1. Working principle
The working principle of sodium-ion batteries is similar to that of lithium-ion batteries, which realizes the storage and release of electrical energy based on the insertion/deintercalation process of sodium ions between the positive and negative electrodes. It mainly includes the following steps:
Charging process: Under the action of an external electric field, sodium ions are deintercalated from the positive electrode material (such as layered oxides or polyanionic compounds), migrate to the negative electrode (such as hard carbon, sodium titanate, etc.) through the electrolyte, and are embedded in the negative electrode material. At the same time, electrons flow from the positive electrode to the negative electrode through the external circuit.
Discharging process: Sodium ions are deintercalated from the negative electrode material, migrate back to the positive electrode through the electrolyte, and electrons flow back to the positive electrode through the external circuit to release electrical energy.
The positive and negative electrode reactions of sodium ion batteries determine the electrochemical performance and energy density of the battery. Typical positive and negative electrode reactions are as follows:
Positive electrode reaction (taking NaFePO4 as an example):
NaFePO4 ↔ FePO4 + Na+ + e-
Negative electrode reaction (taking hard carbon as an example):
C+Na+ + e- ↔ NaC
The reversibility and stability of these reactions directly affect the charge and discharge efficiency and cycle life of the battery.
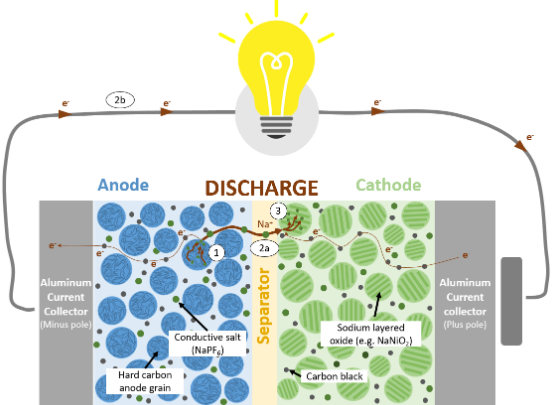
Figure 1 Example of chemical structure of sodium-ion battery (positive electrode: layered oxide, negative electrode: hard carbon) Schematic diagram of discharge process
2. Differences between sodium-ion batteries and lithium-ion batteries
Although the basic working principles of sodium-ion batteries and lithium-ion batteries are similar, there are some important differences in ion insertion and extraction processes, material selection and electrochemical properties:
Ionic radius and mass: The ionic radius of sodium ions (1.02 A) is larger than that of lithium ions (0.76 A), and the mass is also heavier than that of lithium ions. This means that the migration speed and diffusion coefficient of sodium ions are lower, which may lead to the kinetic performance of sodium-ion batteries being inferior to that of lithium-ion batteries.
Electrode material selection: Due to the large radius of sodium ions, some electrode materials suitable for lithium-ion batteries are not suitable for sodium-ion batteries. For example, graphite is not stable in sodium-ion batteries, while hard carbon, oxides and polyanionic compounds perform well in sodium-ion batteries.
Voltage platform: The operating voltage of sodium-ion batteries is generally lower than that of lithium-ion batteries. This is because the standard electrode potential of sodium (-2.71 V vs. SHE) is higher than that of lithium (-3.04 V vs. SHE), so the energy density of sodium-ion batteries is lower.
Electrolyte compatibility: The electrolyte system of sodium-ion batteries needs to adapt to the characteristics of sodium ions, and sodium salts such as NaPF6 or NaClO4 are usually used, while lithium-ion batteries usually use LiPF6 as electrolyte salt.
3. Electrode material selection
The performance of sodium-ion batteries depends largely on the selection of positive and negative electrode materials:
Positive electrode materials: Common positive electrode materials for sodium-ion batteries include layered oxides (such as NaCoO2, NaFeO2), polyanionic compounds (such as Na3V2(PO4)3, NaFePO4) and Prussian blue compounds. The ideal positive electrode material should have high capacity, good cycle stability and low cost.
Negative electrode material: Hard carbon is currently the most commonly used negative electrode material for sodium-ion batteries, with good cycle stability and moderate capacity. In addition, sodium titanate (NaTiO2), metallic sodium and alloys (such as Sn, Sb) have also been widely studied to improve the energy density and rate performance of batteries.
4. Electrolyte
The electrolyte plays the role of transferring sodium ions in sodium-ion batteries. Common types of electrolytes include liquid electrolytes, solid electrolytes and gel electrolytes:
Liquid electrolyte: usually sodium salts (such as NaPF6, NaClO4) dissolved in organic solvents (such as ethylene carbonate, propylene carbonate). Liquid electrolytes have high ionic conductivity, but there are problems of flammability, volatility and poor safety.
Solid electrolyte: including oxides (such as Na-β-Al2O3), sulfides (such as Na3PS4) and polymer electrolytes. Solid electrolytes have high safety and good mechanical strength, but relatively low ionic conductivity.
Gel electrolyte: by adding polymers to liquid electrolytes to form gels, it has both the high ionic conductivity of liquid electrolytes and the high safety of solid electrolytes.
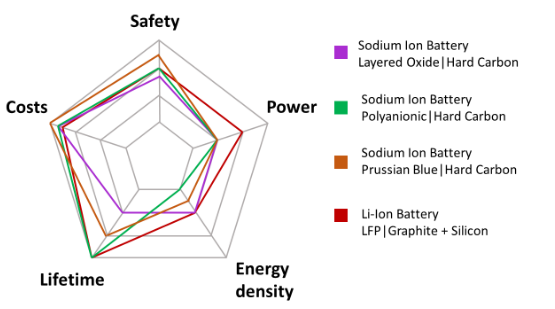
Figure 2 Comparison of different sodium ion chemical properties
II. Failure mechanism
1. Structural changes of electrode materials
During repeated sodium ion insertion/deinsertion, the lattice structure of the electrode material will change, resulting in mechanical stress and volume changes of the material, causing particle rupture and active material shedding, reducing the capacity and cycle life of the battery. At the same time, the electrode/electrolyte interface is one of the key factors affecting the performance of sodium ion batteries. During the cycle, a passivation layer (such as the solid electrolyte interface layer SEI) is easily formed at the interface, resulting in increased interface impedance, affecting ion transport and battery performance. The ideal SEI layer should have high ionic conductivity, good chemical stability and mechanical strength to protect the electrode, prevent further decomposition of the electrolyte and regulate ion transport.
2. Formation of sodium dendrites
In the sodium metal anode, the deposition process of sodium ions may lead to the formation of sodium dendrites, which may pierce the diaphragm in severe cases, causing short circuits and safety problems. The formation of sodium dendrites is mainly affected by current density, surface state of the anode and electrolyte composition. Solutions to the sodium dendrite problem include optimizing current density, improving the surface structure of the anode material and introducing functional electrolyte additives.
3. Degradation of electrolytes
Liquid electrolytes will degrade during long-term use, and the generated byproducts may react with electrode materials and affect the performance of the battery. For example, carbonate solvents are easy to decompose and generate gases under high voltage, leading to battery expansion and leakage problems. Although solid electrolytes perform better in chemical stability, they have poor interface contact with electrode materials, resulting in increased interface impedance and affecting battery performance. An ideal electrolyte should have high sodium ion conductivity and a wide electrochemical stability window. Common electrolytes and their conductive mechanisms include: Liquid electrolytes: Migrate sodium ions under the action of an electric field by solvating sodium ions. Solid electrolytes: Conduct sodium ions through ion channels in the lattice, such as the oxygen ion vacancy migration mechanism in Na-β-Al2O3. Gel electrolytes: Combine liquid electrolytes and solid matrices to form a flexible structure and improve ionic conductivity.
III. Safety issues
1. Thermal runaway
Thermal runaway is one of the most serious safety issues of sodium-ion batteries in high temperature environments. High temperatures can cause electrolyte decomposition, intensified electrode material reactions, and SEI layer failure, generating a large amount of heat and gas, causing battery fires and explosions. Measures to prevent thermal runaway include:
Thermal management system: Introduce efficient thermal management systems such as phase change materials (PCM) and high thermal conductivity materials in battery design to quickly dissipate heat and keep the battery operating within a safe temperature range.
Fire protection design: Add fireproof barriers and flame retardant materials such as fireproof isolation membranes to battery modules to reduce fire risks.
Thermal runaway inhibitors: Add thermal runaway inhibitors such as phosphate compounds to the electrolyte to improve the thermal stability of the electrolyte and reduce the occurrence of decomposition and side reactions.
2. Prevention and treatment of sodium dendrites
The formation of sodium dendrites not only affects battery performance, but may also pierce the diaphragm, causing short circuits and safety problems. Measures to prevent the formation of sodium dendrites include:
Optimize current density: During charging, control reasonable current density to reduce the formation of sodium dendrites.
Improve the surface structure of negative electrode materials: Improve the uniformity of the surface of negative electrode materials and reduce dendrite growth through surface modification and nanostructure design.
Functional electrolyte additives: Introduce functional electrolyte additives, such as ionic liquids and SEI film-forming additives, to regulate the deposition behavior of sodium ions and inhibit dendrite formation.
3. Electrolyte leakage
The leakage of liquid electrolyte is one of the safety hazards faced by sodium-ion batteries in practical applications. Leaking electrolyte not only leads to a decline in battery performance, but may also cause short circuits and fires. Methods to prevent electrolyte leakage include sealing design: In battery design, high-strength sealing materials and structures are used to prevent electrolyte leakage. Solid electrolyte substitution: Use solid electrolytes to replace liquid electrolytes to eliminate leakage risks and improve battery safety.
IV. Future Prospects
Sodium-ion batteries have broad application prospects in the field of energy storage, but many technical challenges still need to be solved before their widespread application can be achieved. Future research directions include:
1. Development of new materials
Continuous exploration of new electrode materials and electrolyte materials, especially materials with wide temperature stability, is the key to improving the performance of sodium-ion batteries. For example, the study of polymer electrolytes and nanocomposites with self-healing functions is expected to provide higher electrochemical performance and stability under wide temperature conditions.
2. Interface optimization
Further research and optimization of the stability and ion transfer efficiency of the electrode/electrolyte interface is an important direction to improve the overall performance of the battery. Forming a low-impedance, high-stability interface through surface modification and interface modification is an important topic for future research.
3. System integration and application
When sodium-ion batteries are applied to actual energy storage systems, the integration and optimization design of battery packs must be considered. By optimizing the battery module design, thermal management system and safety protection measures, the efficient and safe operation of the battery in a wide temperature range can be ensured.
4. Sustainability and economy
In the process of developing sodium-ion battery technology, its sustainability and economy must be considered. For example, low-cost, environmentally friendly electrode materials and electrolytes, as well as efficient recycling and reuse technologies, should be studied to reduce the production and use costs of sodium-ion batteries and improve their economic benefits.
Conclusion
Sodium-ion batteries show great potential in the field of energy storage due to their abundant resources, low cost and good electrochemical properties. However, to achieve the widespread application of sodium-ion batteries, challenges in design principles, failure mechanisms, basic chemistry and safety issues still need to be addressed. Through continuous research and technological innovation, the performance and safety of sodium-ion batteries can be significantly improved, promoting their application in large-scale energy storage and electric transportation. Future research directions include the development of new materials, interface optimization and system integration, as well as sustainability and economic considerations. We look forward to promoting the development of sodium-ion battery technology and realizing the sustainable use of green energy through multidisciplinary cross-collaboration.
Post time: Dec-02-2024
